CROS Reverse Osmosis Systems producing Water for Injection (WFI)
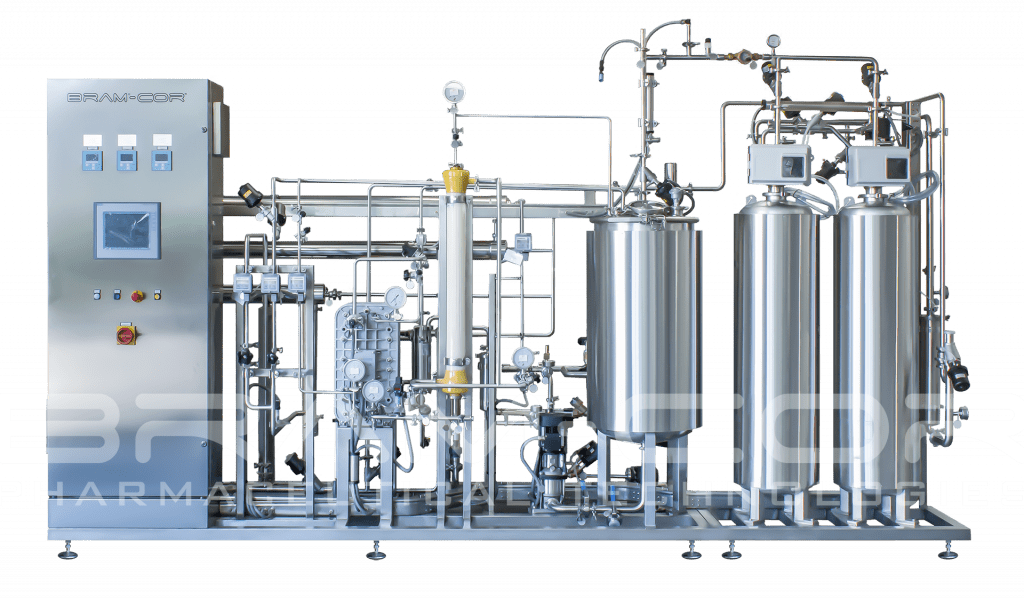
According to the United States Pharmacopoeia, to the Japanese Pharmacopeia and to the European Pharmacopeia, Water for Injections in bulks can be produced with a purification process that is equivalent to distillation.
CROS RO systems, either in SINGLE or DOUBLE PASS, combined with EDI and polishing Ultrafiltration modules, provide a robust production method, yielding cold WFI in compliance with pharmacopoeial quality attributes:
• RO membranes, with a molecular weight cut-off value of 100 Dalton, are able to remove pyrogens and micro-organisms.
• Continuous Electrodeionization removes ionizables substances reducing water conductivity by a combination of ion exchange membranes, ion exchange resins and DC electrical potential.
• Polishing Ultrafiltration is a membrane-based process using a molecular weight cut-off of 6000 Dalton to reduce endotoxins, TOC and bacteria.
All these components are suitable for hot water sanitization, for biofilm control and prevention, in association with appropriate sampling SOPs.
WATER FOR INJECTION IN BULK**update Jan 31 2021 |
||
| PHISICAL / CHEMICAL | Ph. Eur. | USP |
| Appearance | Colorless, clear | Not defined |
| Conductivity | ≤ 1.1 μS/cm@20°C | ≤ 1.3 μS/cm @25°C |
| TOC | ≤ 0.5 mg/L | ≤ 0.50 mg/L |
| Nitrates NO₃ | ≤ 0.2 ppm | Not defined |
| Aluminium | ≤ 10 ppb | Not defined |
| MICROBIOLOGICAL | Ph. Eur. | USP |
| Bacterial count | ≤ 10 CFU/100 ml | ≤ 10 CFU/100 ml |
| Bacterial endotoxins | < 0.25 IU/ml | – |
