A clear vision about purified water generation in cosmetic and biopharma environment
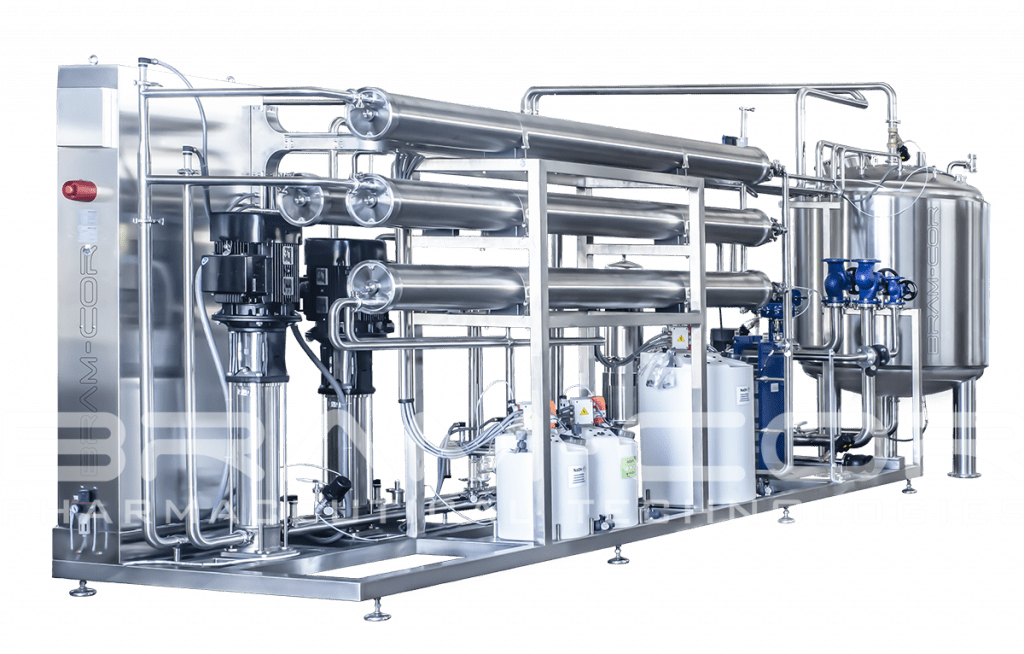
Sanitary Reverse Osmosis Systems
CROS systems, entirely manufactured in Italy, can produce Purified Water (PW) and, with a supplementary step of Ultrafiltration (UF), “cold” Water for Injection (CROW models).
Thanks to different water pretreatment techniques, such as pre-filtration, Multi-Media filtration, softening, pre-treatment Ultrafiltration, chemical dosing, UV irradiation or Granulated Active Carbon, combined with the Reverse Osmosis system with or without Electrodeionization, BRAM-COR RO systems can work with any type of feed water.

Application
- Production of Purified Water (PW, CROS models) and/or Water for Injection (WFI, CROW models) for pharmaceutical/cosmetic use and laboratories.
- Production of Ultrapure Water (UPW) for Semiconductors and Microelectronics.
Technology
- The Reverse Osmosis unit can also use the latest hot water sanitizable advanced membrane technology to remove up to 99% of dissolved inorganics and organics, colloids and particles.
- CROS/CROW are available either in single or twin pass Reverse Osmosis, with additional permeate refining and disinfection systems, such as EDI module, gas stripping station, UV reactor, and Ultrafiltration module, according to the URS.
Techn. Features
- Designed, automated and validated according to GAMP 5, fully compliant with the latest specifications of latest ASME BPE, international GMP, USP and Ph. Eur.
- In-line measurement of process parameters
- Water recirculation during idle periods to prevent biofilm growth
- Centrifugal booster pump in SS with electronic pressure transducers automatically regulating pump capacity
- All surfaces in contact with PW in certified AISI 316L SS / compatible materials according to applications
- Skids and control cabinets in AISI 304 SS
- Orbital and manual welds executed by qualified welders
- Full traceability of all components
- Sampling points after each treatment step
- • Commissioning & qualification package (FAT, IQ, OQ)
- Can be combined with customized feedwater pretreatment systems and with PW or cold WFI storage and distribution system.
Sanitization
- Reduction of microbial contamination through regular hot water sanitization.
- Sanitization of the RO System can be performed by chemical cleaning or hot water recirculation at ≥70°C (option)
Control System
Functions operated by the PLC (Programmable Logic Controller):
-
- Input of measured values and setting of limit values
- Automatic Sequences (production, sanitization, …)
- Control Functions (PID control for valves and speed of pump)
- Alarm management and Verification of parameters
- Input of measured values and setting of limit values
- Output commands for digital and analogic values
HMI System
HMI (Human Machine Interface)
- Display of machine state
- Controls management
- Verification of alarms
- Set points inserting and limit values setting
- Graphic interface
All automation systems can be in compliance to 21 CFR PART 11 or Siemens Operator Panel, through audit management and electronic signature. Access management included (user/password).
SCADA System
SCADA (Supervisory Control And Data Acquisition) / SCADA SERVER
-
- All HMI values and controls
- Data historicization
- Historical alarms
- Trend and Report
- Recipes formulation / Batch
- Data backup / Restore
All automation systems can be in compliance to 21 CFR PART 11 or Siemens Operator Panel, through audit management and electronic signature. Access management included (user/password).
Communication
Bram-Cor automation systems can virtually communicate with all network partners through maximum security protocol (Ethernet, Profinet, OPC Unified Architecture, …)
Options:
-
- Teleservice (malfunctions managed remotely by Bram-Cor)
- Remote Control (customer operator receives a message / a text message / a warning e-mail)
- Server-side data centralization (customer can centralize data on his service, or Bram-Cor provides it)

CROS CUSTOMIZABLE CONFIGURATIONS

CROS SINGLE PASS - Sanitary Reverse Osmosis System + EDI Electrodeionization
CROW SINGLE PASS - Sanitary Reverse Osmosis System + EDI Electrodeionization + UF Ultrafiltration

CROS DOUBLE PASS - Sanitary Reverse Osmosis System

CROS DOUBLE PASS - Sanitary Reverse Osmosis System + EDI Electrodeionization
CROW DOUBLE PASS - Sanitary Reverse Osmosis System + EDI Electrodeionization + UF Ultrafiltration

FULL GAMP AUTOMATION
CROS/CROW systems are managed through GAMP compliant hardware & software.
PHARMACEUTICAL REVERSE OSMOSIS SYSTEMS PRODUCING PURIFIED WATER (PW)
 CROS SIN/DOP/EDI: RO Systems producing Purified Water (PW)
CROS SIN/DOP/EDI: RO Systems producing Purified Water (PW)
Purified Water generation by CROS systems, as defined by USP, Ph Eur, JP, ChP and IP monographs, is classified for pharmaceutical use as an excipient in the production of non-parenteral preparations and for other pharmaceutical applications, such as for CIP cleaning of production equipment and for washing of non-parenteral product-contact components.
PHARMACEUTICAL REVERSE OSMOSIS SYSTEMS PRODUCING WATER FOR INJECTION (WFI)

CROW SIN/DOP EDI + UF: RO Systems producing Water for Injection (WFI)
According to the United States Pharmacopoeia (USP), the Japanese Pharmacopeia (JP) and the European Pharmacopeia (Ph.Eur.), Water for Injections in bulks can be produced with a purification process that is equivalent to distillation.
CROS DESIGN
Bram-Cor engineering satisfy all pharmaceutical regulatory and QA requirements
From design to manufacturing, CROS/CROW systems are aligned with the international cGMP and Pharmacopoeias.
Bram-Cor Reverse Osmotic equipment is endowed with a Recycling system of the concentrate in order to obtain a higher recovery with a lower consumption of inlet water.

Bram-Cor RO systems
full documentation
BRAM-COR CROS/CROW documentation is composed by:
• GMP collection of plant-specific drawings, technical specifications, materials certificates, calibration certificates, hardware and software specifications, welding documentation, plant manuals (TECHNICAL DOCUMENTATION);
• DATASHEETS & MANUALS BOOK, containing all the datasheet and manuals of the commercial components (valves, instruments, pumps, etc) installed on the equipment.
REVERSE OSMOSIS SYSTEMS
Bram-Cor manufacturing excellence in every standard – Customized design for any application

Pharmaceutical Equipment manufacturing
Bram-Cor provides the highest quality. cGMP, PED standards and ASME S/U certification on one side and international pharmacopoeias on the other, are the baseline criteria for ourdesign and construction. All Bram-Cor biopharma processing systems ensure full traceability, certified material and instruments.


Professional Services
Bram-Cor equipment is integrated through a proper high level of professional services including:
- Technical Documentation
- Commissioning
- Installation
- Start-up and Training
- Validation (Equipment Qualification)

Worldwide Assistance
Bram-Cor machines are delivered all over the world and are supported by Bram-Cor After Sales service. Bram-Cor worldwide network ensures assistance in over 50 countries, from the very beginning of a pharmaceutical project and for decades after start-up. Bram-Cor After Sales dept. provides punctual and quick deliveries of spares and ongoing technical support.

PW, WFI and Compendial waters
BRAM-COR engineering focuses on liquid / sterile drug and low / medium / high viscosity production processes, such as parenteral solutions, oral solutions, ophthalmic and oncology solutions, low / medium / high viscosity emulsions, cosmetic preparations. Purified Water (PW) and Water for Injection (WFI) are the obligatory basis of many pharmaceutical preparations.
Bram-Cor Catalogue
Pharmaceutical Equipment
BRAM-COR Pharmaceutical Equipment General Catalogue. In this thirty-six pages brochure, you can discover “at a glance” BRAM-COR systems and plants.

 CROS SIN/DOP/EDI: RO Systems producing Purified Water (PW)
CROS SIN/DOP/EDI: RO Systems producing Purified Water (PW)